Impact of Lifestyle & Aging Mitochondria on Longevity and Wellness
You may recall mitochondria being referred to as the powerhouses of the cell from your high school biology class. This is because these tiny organelles (mini organs), present in all human cells (except red blood cells), are responsible for turning food into energy in the form of Adenosine triphosphate (ATP) — the cellular currency of energy. For a deep dive into mitochondrial function, check out an earlier blog on this website.
The role of mitochondria in an individual’s health, longevity, and wellness is actually much more multifaceted and nuanced than the maintenance of bodily functions and energy supply. In this sense, one can understand that mitochondrial function is not a 0 – 1 function, as in, it works (great) versus it’s broken (you die)
In this article on the impact of lifestyle and aging mitochondria on longevity and wellness, let’s start with the beginning of the energy cycle, namely, the consumption of food (calories) and its transformation at three levels:
- Systemic (whole body) level
- Organ level
- Cellular level
Calorie Intake at the Systemic (Whole Body) Level
It is true that a person depends on a certain amount of calories to survive (and thrive), but the kinds of foods used to fulfill these calorie needs is also important. For example, research has shown that unprocessed foods are usually better than processed foods due to their lower calorie density and higher content of vitamins, micronutrients, fiber, and the macronutrient balance.[1]
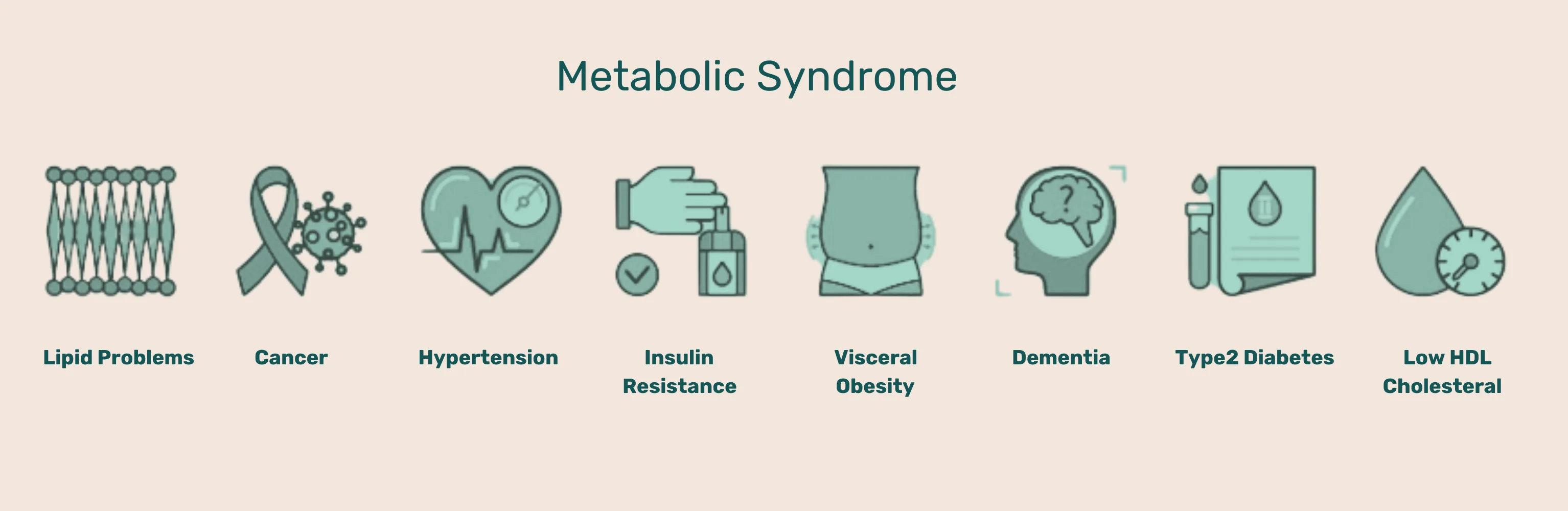
Besides the type of calories consumed, the number of calories consumed is also important; eat too much and it can result in being overweight or even obese. Obesity can lead to metabolic stress which can manifest in non-communicable diseases (NCDs), such as Type II diabetes, cardiovascular diseases, cancers, chronic respiratory diseases, inflammatory diseases, among others.
It is important to keep in mind that every body benefits from a balance that includes carbohydrates and fats, despite what the diet fads of the day may purport. Therefore, healthier foods in a measured intake are key to making you feel good as a human.
Digestion of Nutrients at the Organ Level
Depending on an individual’s particular genetic and cellular makeup, they may or may not tolerate certain types of food contents, such as lactose or gluten. Allergies and other food sensitivities can cause adverse reactions such as inflammation at the location of their uptake, for example, in the stomach or colon. These in turn can further reduce the absorption of key nutrients.
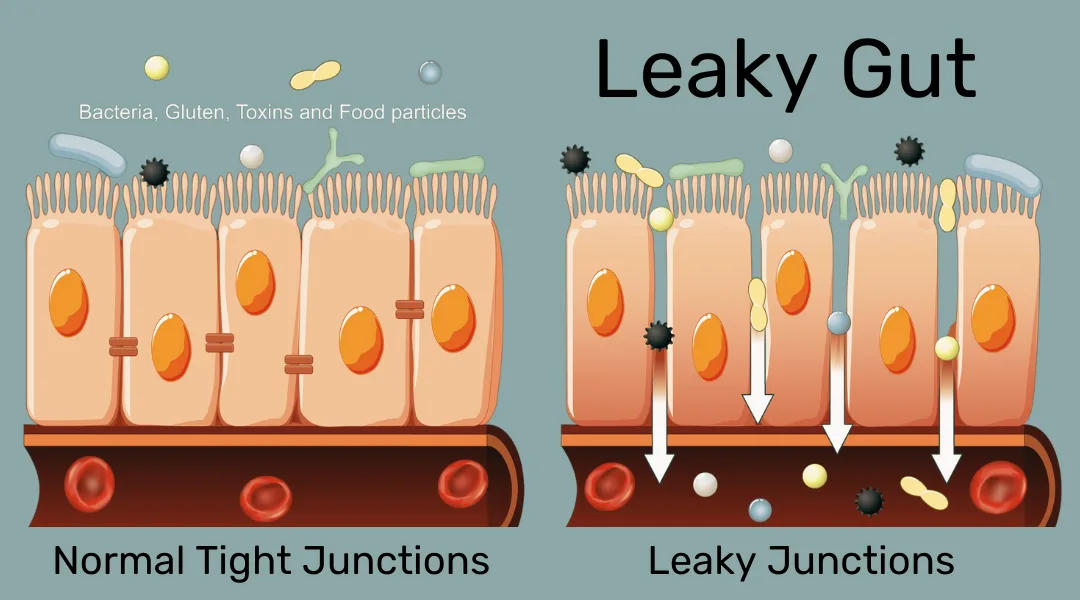
The digestion process starts in the mouth with the mechanical breakdown of food and enzymes, such as amylase which attack long-chain carbohydrates like starch. The stomach is mainly responsible for the digestion of proteins, and the small intestine together with the pancreas and gallbladder take care of everything else, especially fats.
The macronutrients are broken down into glucose (from carbohydrates), peptides or amino acids (from proteins) and fatty acids and monoglycerides (from fats).
The uptake in the small intestine (specifically the duodenum and jejunum) depends on intestinal health, so it is important to pay attention to personal nutritional sensitivities to avoid inflammation and allow for optimal energy generation from food.
Conversion of Energy at the Cellular Level
Glucose, amino acids, and fatty acids enter cells via transporters, specifically, the GLUT4, active and passive amino acid transporters and fatty acid binding proteins, respectively. Once inside the cells, glucose is stripped of as much energy as possible during glycolysis, and the remaining pyruvate then enters the mitochondria.
Amino acids, if possible, get repurposed for protein synthesis, or can enter the mitochondria via the metabolic TCA cycle. Mainly, however, mitochondria feed off of fatty acids which are transported in via the carnitine shuttle to undergo beta-oxidation.
And here, at the cellular level, is where mitochondrial health really comes into play.
Acetyl-CoA generated from carbohydrates, fats and proteins is shuttled into the TCA cycle, a metabolic cycle that supplies various re-usable intermediates for energy metabolism. Imagine breaking an interlocking brick house or a car down to its basic pieces, and then reusing the basic pieces to build new structures and use as needed in the moment.
The TCA cycle produces the intermediate energy storage molecules NADH and FADH2, which store electron potential — they have the ability to donate electrons and engage in reduction reactions with other molecules. This is used to feed into the electron transport chain, which generates the cellular energy currency, ATP, through oxidative phosphorylation. Basically, this is like a system of stepwise waterfalls and pumps, which are powered by the waterfalls and put out energy, in the form of ATP, as a result.
ATP is the fuel that the cell uses for all of its energy needs — movement, stability, growth, and regeneration.
Metabolism at the systemic level and actual cellular energy production in the mitochondria of the cells are closely intertwined and related. If any step along that process falters, energy available to the cells and, therefore, to you, is reduced.
Mitochondria are Not Static
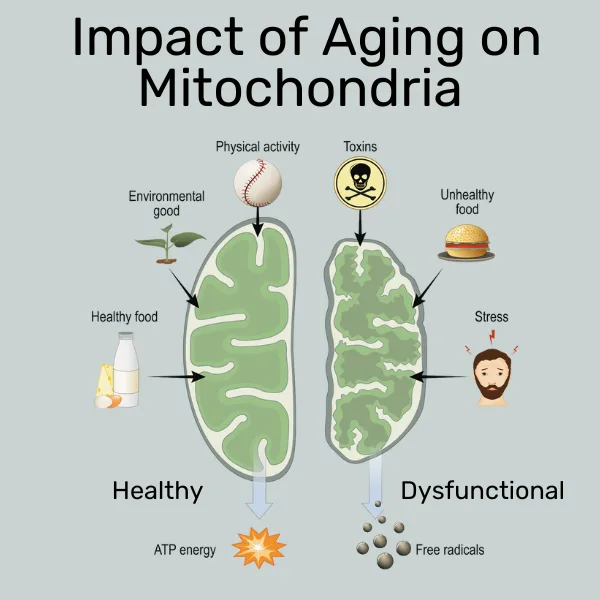
Mitochondria are an ever-evolving, dynamic network that continually adjusts to outer circumstances. For example, under energy scarcity conditions (slight energy deficit or fasting), the mitochondria band together (fusion), which makes them more efficient and enhances oxidative phosphorylation and energy output.
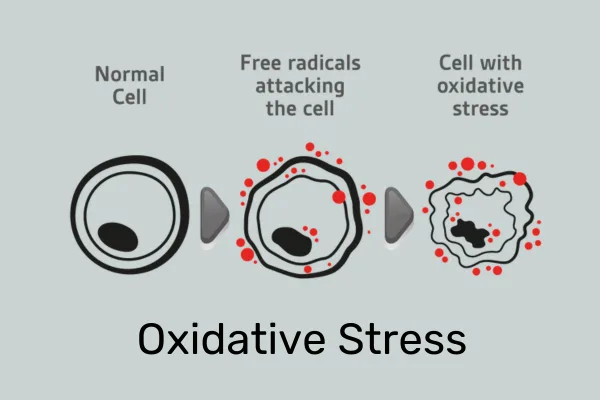
Under adverse circumstances or overnutrition, mitochondria break apart (fission), which leads to decreased efficiency of the oxidative phosphorylation that leads to energy generation, and increased levels of reactive oxygen species (ROS), commonly known as free radicals. These free radicals have been implicated in DNA damage and a range of negative effects on health.
When taken to the extreme, this impaired function can lead to full-blown mitochondrial dysfunction, which has been associated with a range of diseases, especially in the early stages of pathogenesis (disease generation). For example,
- In Alzheimer’s and Parkinson’s, the mitochondria in the neurons are small and fragmented (hinting at fission), which may be a cause or a result of their nervous system dysfunction.
- In metabolic diseases, like non-alcoholic fatty liver disease (NAFLD), the mitochondria show fission and an increase of the cellular upregulators.[2]
There is growing evidence on the importance of mitochondrial health and efficiency of cellular energy generation, and how they can be both the cause and the consequence of life-altering chronic conditions.
Role of Mitochondrial Dysfunction in Aging
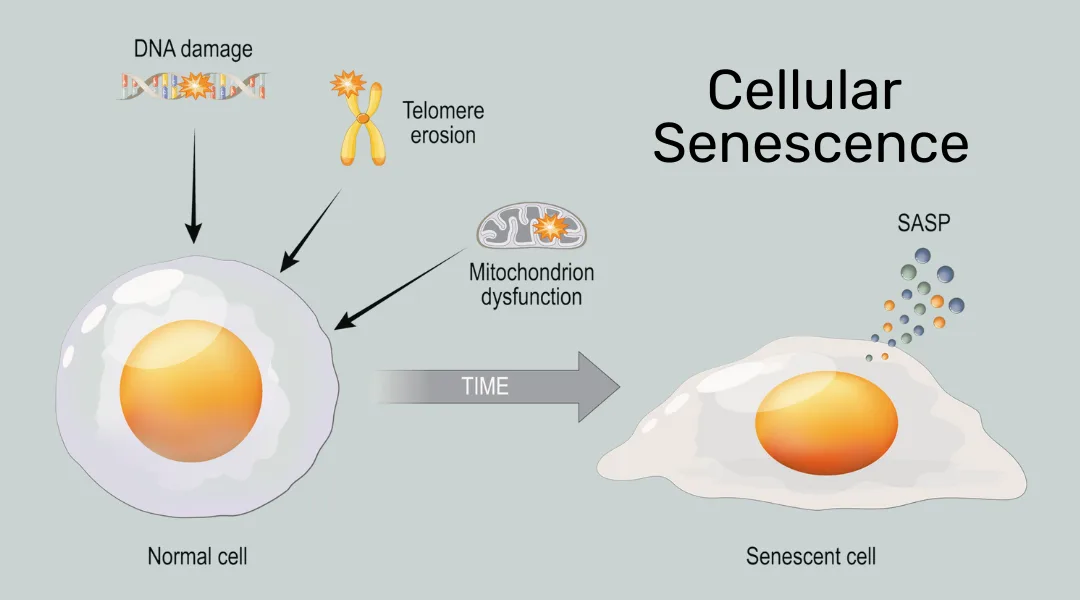
Mitochondrial dysfunction does not just occur during serious illnesses; it also occurs as part of the natural aging process that all individuals go through. Mitochondria in aging cells often exhibit accumulation of mutations in their mitochondrial DNA (mtDNA) and these have been associated with reduced energy generation capacity and are seen as a hallmark of aging cells.[3]
Additionally, cellular signals coming from the nucleus via the protein PARP1 can also negatively impact mitochondrial health in aging organisms, including humans.
When Aging Leads to Cell Senescence
At its extreme, aging leads to cellular senescence, a state in which cells can no longer progress in the cell cycle or divide, and change their gene expression towards a pro-inflammatory signature. Accumulation of senescent cells is a major driver of the aging process and chronic inflammation. See an earlier article on this blog for a more detailed read on chronic inflammation.
The senescent state is characterized by deceased oxidative phosphorylation potential in the early stages, followed by decreased mitochondrial membrane potential and increased proton leakage. Like a leaky pipe, this reduces the efficiency of cellular energy production. In addition, more ROS are produced which can in turn lead to more DNA mutations, general oxidative damage, and chronic inflammation.
Besides the phenomenon of mitochondria performing less well in an aging individual, the number of mitochondria per cell has been shown to decline with age in animal and human studies.[4]
Impact of Metabolic Stress on Cellar Aging
We now understand that both diet and age influences energy levels and wellness, all the way down to the mitochondria within the cells.
But how is this interrelated? The answer is simple: Metabolic Stress!
Metabolic stress is defined as a mismatch of the metabolic needs of an organism and the metabolic input, processing and resulting energy output that the body can provide based on among other things, its mitochondrial efficiency.
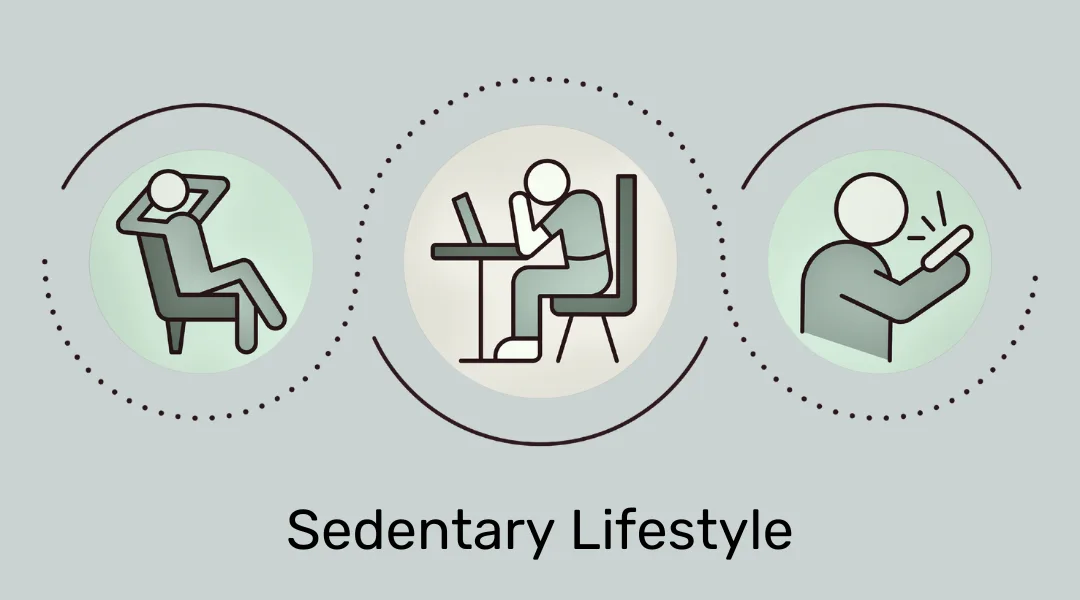
This mismatch of energy needs and production can result from both an energy deficiency, or an excess of it. In most of the Westernized world, the excess of energy is drastically more prevalent because many people eat very high caloric diets and lead mainly sedentary lifestyles; for example, being deskbound for 40 or more hours a week doing knowledge work.
It turns out that rise of processed foods and high-calorie diets coupled with reduction in physical activity due to urbanization has become so prevalent around the globe that according to the World Health Organization, the epidemics of Type II diabetes and obesity have reached a new, all-time high of 537 million adults and 2.5 billion adults affected, respectively, in 2021 and 2022. Vascular diseases and NAFLD are also increasingly on the rise.
Even in individuals considered still healthy — not officially diagnosed with metabolic syndrome (the precursor to Type II diabetes or obesity) — the Western lifestyle may have already taken its toll on cellular health, particularly the mitochondria, resulting in accelerating aging.
Mitochondrial Dysfunction in Accelerated Aging
Studies show that decreased mitochondrial oxidative capacity and a reduction in mitochondrial density in cells are more common in individuals with obesity and insulin resistance as compared to lean, healthy individuals.[4]
Decreased lipid oxidation through mitochondria in white fat cells can contribute to ectopic storage of fat, meaning that the fat is stored in places in the body where it doesn’t belong, for example, in the liver, leading to NAFLD.
Stressed or damaged mitochondria release their mtDNA, which functions as an alarmin — a strong activator of the innate immune system, which via the NF-kB signaling pathway then leads to non-specific, chronic inflammation. Additionally, ROS released from mitochondria activate the so-called inflammasome, leading to a pro-inflammatory cellular environment.
These negative consequences of mitochondrial stress can lead to chronic inflammation, which is in turn associated with chronic conditions often associated with aging, such as rheumatoid arthritis, arteriolosclerosis, NAFLD, obesity and Type II diabetes.
In short: The typical Western lifestyle is speeding up the decline of mitochondrial function during aging. What this means is aging at an advanced rate with a downward spiral being created with increased mitochondrial decline, reduced energy levels, and increasing levels of chronic inflammation.
So how can an individual break out of this downward spiral?
Reducing the Negative Effects of Lifestyle on Mitochondria
Some of the key ways of reducing accelerated aging and damage to the mitochondria include:
- Paying attention to diet and calorie intake, especially processed versus unprocessed foods
- Exercising regularly to keep up muscle mass as well as aerobic efficiency
- Reducing physical and mental stress
- Getting a proper night’s sleep
Read Next
- Salugenesis – Body’s innate healing process
- Chronic inflammation and mitochondria
- Mitochondrial dysfunction in autoimmune and inflammatory disorders
References
- Marti A. Ultra-Processed Foods Are Not “Real Food” but Really Affect Your Health. Nutrients. 2019 Aug 15;11(8):1902. Ultra-Processed Foods Are Not “Real Food” but Really Affect Your Health
- Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Sig Transduct Target Ther 8, 333 (2023). Mitochondrial dynamics in health and disease
- Amorim, J.A., Coppotelli, G., Rolo, A.P. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol 18, 243–258 (2022).
- Ana Bratic, Nils-Göran Larsson. J Clin Invest. 2013;123(3):951-957. The role of mitochondria in aging



