Role of Menopause & Mitochondria in Women’s Aging
Estrogen is a pivotal category of hormones in the female body. A sex hormone, this category of steroid hormones affects both reproductive and non-reproductive health and is key for healthy female longevity.
Let’s first take a look at the biochemistry behind estrogen. Contrary to popular belief, there is not just one estrogen – estrogen is a category of sex hormones consisting of three primary forms of estrogen:
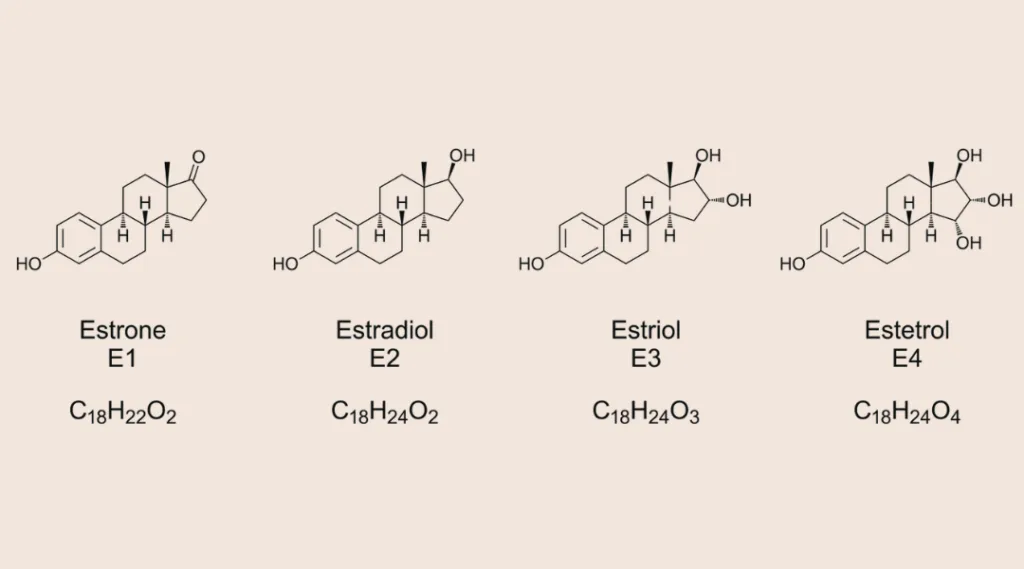
Estrone (E1): Predominant post-menopause, it is primarily produced in fat tissue (adipose tissue).
Estradiol (E2): Most potent form of estrogen, regulating menstrual cycles and supporting reproductive tissues. The lack of E2 really hits hard after menopause.
Estradiol (E3): Primarily produced during pregnancy and important for fetal development and the health of the mother.
Estetrol (E4): Also produced during pregnancy in the liver of the fetus.
Pre-menopause, estrogen is primarily made in the ovaries. Post-menopause, most production shifts to peripheral tissues, such as the fat tissue (adipose tissue) and the adrenal glands. These tissues produce estrogen through modifying androgens, the other type of sex hormone prevalent in both males and females but dominant in males.
Estrogen is key to Bone, Heart, and Skin Health
Most people are aware that estrogen is essential for a female body’s reproductive function. It controls the growth of the uterine lining, stimulating its thickening during the cycle. If no fertilization with implantation of the embryo occurs, estrogen levels drop sharply and the uterine lining is shed during menstruation.
Estrogen also regulates both a negative and a positive feedback cycle during the menstrual cycle:
- Low levels of estrogen at the beginning of the cycle signal to the hypothalamus in the brain to release gonadotropin-releasing hormone (GnRH), which in turn stimulates the pituitary gland to secrete Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH).
- As estrogen levels rise, they inhibit this release during the progression of the cycle, to allow follicles to grow. Mid-cycle, estrogen surges and, now through a positive feedback cycle, triggers a spike in LH and FSH, inducing ovulation.
Less commonly known is the key role played by estrogen in the following non-reproductive health areas:
- Maintains bone density by inhibiting its breakdown (bone resorption) and promotes bone health by enhancing calcium absorption in the gut and regulating levels of active vitamin D.
- Promotes cardiovascular health by shifting the balance of cholesterol towards the good HDL, widening blood vessels via nitric oxide, and reducing inflammation, which helps prevent atherosclerosis.
- Stimulates collagen production, which is essential for skin elasticity and a young look, as well as mood booster effects via their interaction with serotonin. In about 5% of females, the downside of this is observable in Premenstrual dysphoric disorder (PMDD), with excessive mood drops and swings.
Three Stages of Menopause: Perimenopause, Menopause, and Postmenopause
The key takeaway from the previous section is that there is a stark difference between the time before and the time after menopause. But what exactly is menopause, anyway?
Let’s have a look.
Towards the end of a female’s reproductive years, usually between 40 and 50 years of age, hormone levels change, which may cause side effects such as hot flashes, anxiety, and brain fog. This start of the process is termed Perimenopause.
After 12 months without menstruation, menopause is confirmed. This is the official end of the reproductive cycles.
Postmenopause is the transition phase in the 12 months after an individual’s last period to the hormonal state they will be in for the rest of their life.
Loss of Estrogen disrupts Mitochondrial Health
Besides being a key player in the transition from the reproductive lifespan through perimenopause and menopause to postmenopause and beyond, estrogen is involved in women’s aging in another important way: by affecting mitochondrial health.
Receptors mediating estrogen’s effects (estrogen receptors, ERs) are present not only in the cell membrane and nucleus of human cells, but also in mitochondria. Thus, it’s no surprise that estrogen is also critical in mitochondrial function, energy production and biogenesis.
As described in another blog article (mitochondria 101), mitochondria power the cell by running metabolites through the electron transport chain, which generates cellular energy currency, ATP.
Estrogen upregulates, which means it increases components of the electron transport chain,and thus the body’s energy supply. It also reduces the formation of reactive oxygen species (ROS) or free radicals, while mitigating aging-promoting oxidative stress.
Thus, it makes sense that after menopause, when estrogen levels drop, mitochondrial function declines as well, with all the resulting effects described in the above.
In fact, this has been shown in several research studies, including in ovariectomized rats. These rats, which had their ovaries taken out and were compared to a sham-operated control group, showed decreased mitochondrial function as evidenced by lower oxygen uptake and ATP production.
Similar effects have been found in (less invasive) studies in women.
Female Brain Aging connected to reduced Estrogen and Damaged Mitochondrial Function
But what about the type of aging-related diseases that for many are the scariest, as they are a direct threat to who we are or our personality – neurodegenerative diseases?
Again, estrogen plays a role in these.
In fact, the brain can synthesize its own estrogen through a process called neurosteroid genesis. In contrast to the estrogen produced in ovaries which is primarily responsible for sexual function, the estrogen synthesized locally in the brain mainly affects cognitive function and provides neuroprotection. It accomplishes this by promoting synaptic plasticity (the ability of your brain to change and rewire itself), and reduces inflammation.
Additionally, it also provides the same protective and pro-functional effects it has on mitochondria in the rest of the body to mitochondria in neurons, ensuring their continuous energy supply. These are particularly crucial in the brain, considering that the brain uses more energy than any other organ in the human body.
All of these neuroprotective functions help prevent neuronal damage and are thus key in reducing risks of Alzheimer’s and Parkinson’s disease.
It makes sense that these neuroprotective functions are hampered when estrogen levels drop after menopause. Unfortunately, this deterioration coincides with the natural decrease in mitochondrial function during aging, which already reduces the efficiency of the electron transport chain, glucose metabolism and ATP production.
In addition, estrogen also stimulates secretion of brain-derived neurotrophic factor (BDNF), which is essential for neuronal survival, synaptic plasticity and cognitive function. It increases the activity of an enzyme called SIRT3, which resides in mitochondria and is involved in cellular health and longevity.
The drastic drop in estrogen after menopause thus contributes to the aging process in the female brain, as underscored by studies showing that women who had oophorectomies (surgery to remove the ovaries) show elevated risk to suffer from cognitive impairment or dementia later in life.
Animal studies have shown strong evidence that mitochondrial dysfunction precedes cognitive defects, thus implying that mitochondrial dysfunction could be the direct cause of the cognitive decline observed.
Connection between Menopause and Cardiovascular Disease Risk
Anti-inflammatory and antioxidant properties of estrogen also contribute to its protective role in aging-related diseases, such as cardiovascular disease (CVD). A study from the American College of Cardiology showed that pre-menopause, women benefit from the cardio-protective effect of estrogen, while after menopause, they rapidly catch up to men with similar profiles in terms of their coronary artery calcium (CAC) score, which predicts susceptibility to cardiovascular issues.
So what’s the mechanism of this sudden deterioration of cardiovascular health in post-menopausal, aging women?
In pre-menopause, estrogen increases the healthy cholesterol HDL while lowering the less favorable LDL and the lack of estrogen after menopause drives up the propensity for CVD. In addition, other parts of lipid metabolism are also disrupted, driving up cholesterol and triglyceride levels and favoring a redistribution of body fat toward abdominal fat. All of these factors are associated with an increased risk for insulin resistance, inflammation and ultimately, diabetes.
At the intracellular level, estrogen deprivation hits heart cells directly: Cardiomyocytes rely heavily on fatty acid metabolism which happens in – you guessed it – mitochondria. Lack of estrogen and the associated disruption of mitochondrial function leads to accumulation of lipid metabolites in the cardiomyocytes, which may cause lipotoxicity and contribute to increased cardiovascular risk.
Improving Mitochondrial Health for Healthier Aging after Menopause
All of this looks like a rather dark outlook for aging women, right? But realistically, this is just a normal part of life, and aging really is what you make of it. So don’t be discouraged. Let’s take a proactive look at what you can do to improve your aging experience before, during and after menopause.
Here are three key strategies to offset the drop in mitochondrial health and functioning due to the lack of estrogen by finding other ways of boosting your mitochondrial function:
- Regular physical activity: Consistent aerobic exercise and strength training has been shown to enhance mitochondrial biogenesis and efficiency.
- Balanced diet rich in antioxidants: Focus on a diet abundant in fruits and vegetables which contain antioxidants, to offset the reduced antioxidative capacity of aging mitochondria.
- Caloric restriction and intermittent fasting: Experiment with these interventions to tackle the metabolic effects observed during estrogen withdrawal after menopause.
Read Next
References
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003 Sep;86(3-5):225-30. doi: 10.1016/s0960-0760(03)00360-1. PMID: 14623515.
- A Guide to Perimenopause, Menopause, and Postmenopause. Sept 30, 2021. School of Nursing, Georgetown University. https://online.nursing.georgetown.edu/blog/a-guide-to-perimenopause-menopause-and-postmenopause/
- Mooga, V. P., White, C. R., & Giordano-Mooga, S. (2018). Estrogen and Mitochondrial Function in Disease. InTech. doi: 10.5772/intechopen. 73015.
- Zárate, S., Astiz, M., Magnani, N., Imsen, M., Merino, F., Álvarez, S., Reinés, A., & Seilicovich, A. (2017). Hormone deprivation alters mitochondrial function and lipid profile in the hippocampus. Journal of Endocrinology, 233(1), 1-14. Retrieved Nov 12, 2024, from https://doi.org/10.1530/JOE-16-0451
- Lejri Imane , Grimm Amandine , Eckert Anne. Mitochondria, Estrogen and Female Brain Aging. Frontiers in Aging Neuroscience. Vol10, 2018. https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2018.00124. DOI=10.3389/fnagi.2018.00124. ISSN=1663-4365
- Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013 Mar;19(3):197-209. doi: 10.1016/j.molmed.2012.12.007. Epub 2013 Jan 22. PMID: 23348042; PMCID: PMC3595330.
- Lejri I, Grimm A, Eckert A. Mitochondria, Estrogen and Female Brain Aging. Front Aging Neurosci. 2018 Apr 27;10:124. doi: 10.3389/fnagi.2018.00124. PMID: 29755342; PMCID: PMC5934418.
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res. 2010;182:77-96. doi: 10.1016/S0079-6123(10)82003-5. PMID: 20541661; PMCID: PMC5776041.
- Zhao Wei , Hou Yue , Song Xinxin , Wang Lei , Zhang Fangfang , Zhang Hanting , Yu Haiyang , Zhou Yanmeng. Estrogen Deficiency Induces Mitochondrial Damage Prior to Emergence of Cognitive Deficits in a Postmenopausal Mouse Model. Frontiers in Aging Neuroscience. Vol 13, 2021. https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2021.713819. DOI=10.3389/fnagi.2021.713819. ISSN=1663-4365
- Ishaaya, E, Kinninger, A, Schwarzman, L. et al. CAC PROGRESSION IN MEN AND WOMEN: IS THERE AN INFLECTION AT MENOPAUSE?. JACC. 2024 Apr, 83 (13_Supplement) 1942. https://doi.org/10.1016/S0735-1097(24)03932-9
- Samar R. El Khoudary, PhD, MPH, FAHA, Chair, Brooke Aggarwal, EdD, MS, FAHA, Theresa M. Beckie, PhD, FAHA, Howard N. Hodis, MD, FAHA, Amber E. Johnson, MD, MS, MBA, Robert D. Langer, MD, MPH, FAHA, Marian C. Limacher, MD, FAHA, JoAnn E. Manson, MD, DrPH, FAHA, Marcia L. Stefanick, PhD, FAHA, Matthew A. Allison, MD, MPH, FAHA, Vice Chair, On behalf of the American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Lippincott Williams & Wilkins. ISSN0009-7322eISSN1524-4539. December 22, 2020. Pagese506 – e532. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association
- Oliveira, Paulo J., Carvalho, Rui A., Portincasa, Piero, Bonfrate, Leonilde, Sardao, Vilma A., Fatty Acid Oxidation and Cardiovascular Risk during Menopause: A Mitochondrial Connection?, Journal of Lipids, 2012, 365798, 12 pages, 2012. https://doi.org/10.1155/2012/365798
- Ju Wenhan , Zhao Yuewen , Yu Yi , Zhao Shuai , Xiang Shan , Lian Fang. Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions. Frontiers in Endocrinology. Vol 15. 2024. https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2024.1361289. DOI=10.3389/fendo.2024.1361289. ISSN=1664-2392
- Lettieri-Barbato D, Cannata SM, Casagrande V, Ciriolo MR, Aquilano K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS One. 2018 May 9;13(5):e0195912. doi: 10.1371/journal.pone.0195912. PMID: 29742122; PMCID: PMC5942780.



