Role of Mitochondria in Arthritis
The term inflammatory arthritis usually refers to a group of arthritis forms in which inflammation of the joints is brought on by an aberrant immune response. This immune-mediated mechanism may result in joint degeneration over time as well as discomfort, edema, and stiffness.
Although Rheumatoid Arthritis (RA) is one of the most well-known types, inflammatory arthritis encompasses a range of illnesses and each of these illnesses has unique causes and symptoms.
Viral Arthritis vs. Inflammatory Arthritis vs. Autoimmune Arthritis
Initially, certain viruses, including parvovirus, hepatitis B and C, Epstein-Barr virus, and viruses spread by mosquitoes, such as Zika and chikungunya, can cause viral arthritis. Acute joint pain and swelling in several joints (polyarthritis) are common presentation symptoms, which frequently resemble those of autoimmune disorders. However, viral arthritis usually goes away in a few weeks and is milder.
Diagnosing viral arthritis is important to differentiate it from other rheumatological conditions, as treatment typically focuses on symptom relief rather than immune suppression.
Another type of inflammatory arthritis called reactive arthritis develops as a reaction to an infection, usually in the urinary or digestive tract. The joints, eyes, and urinary tract may be impacted by this kind of arthritis. It can be difficult to diagnose because the inflammation typically appears after the infection has disappeared. Common symptoms include inflammation where tendons attach to bones (enthesitis) and discomfort in the heel, and especially in the knees and lower back.
Even if the symptoms could go away on their own, the goal of treatment is to control inflammation and avoid consequences.
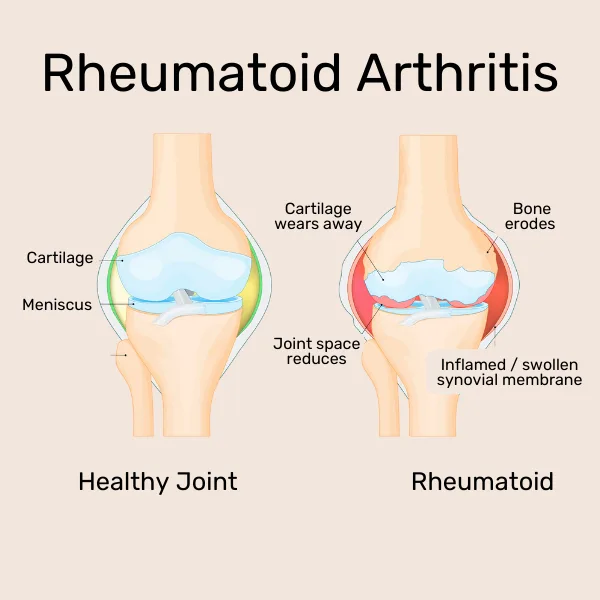
The third type of arthritis, the autoimmune disease rheumatoid arthritis (RA) is caused by the immune system attacking the joint lining (synovium) by mistake, resulting in persistent inflammation. Small joints, such as those in the hands and feet, are the main targets of RA, which is frequently symmetrical, affecting the body equally on both sides.
Untreated RA can result in severe disability and joint abnormalities. Among inflammatory arthritis, RA is distinct due to its progressive and chronic character, which frequently necessitates long-term medication with disease-modifying antirheumatic medications (DMARDs) to prevent joint destruction.
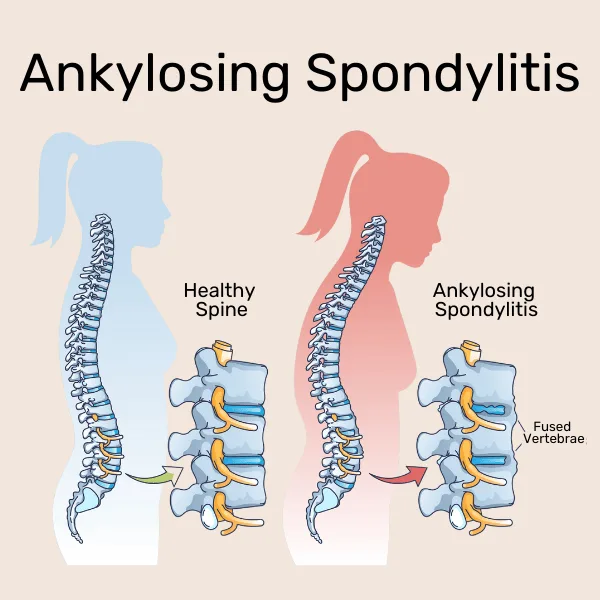
Additional types of inflammatory arthritis include ankylosing spondylitis (AS), which mostly affects the spine and sacroiliac joints, causing stiffness and lower back pain, and psoriatic arthritis (PsA), which is associated with psoriasis. The most common symptom of gout, which is brought on by uric acid crystals accumulating in the joints, is abrupt, excruciating big toe pain.
Rheumatoid arthritis is one particular form of immune-driven joint disease that falls under the general term inflammatory arthritis. Chronic, symmetrical joint involvement and the possibility of irreversible damage if treatment is delayed are the hallmarks of RA.
Certain forms of inflammatory arthritis, such as reactive and viral arthritis, are frequently self-limiting or treated in a different way. They can be brought on by infections. Determining the best course of action for management and treatment requires an understanding of these distinctions.
Role of Mitochondria in Arthritis
As we previously discussed, the term inflammatory arthritis refers to a group of diseases that cause inflammation in the joints, such as gout, psoriatic arthritis, and rheumatoid arthritis (RA). The function of mitochondria, which are organelles in charge of energy production and cellular health, is an emerging element connecting these disorders together.
Recent research has demonstrated that the inflammation and joint destruction typical of many types of arthritis are mostly driven by mitochondrial malfunction.
Mitochondrial Dysfunction in Rheumatoid Arthritis
One of the main causes of inflammation in rheumatoid arthritis (RA) is mitochondrial dysfunction.
- Patients with RA experience oxidative stress due to excess reactive oxygen species (ROS) produced by the mitochondria of their immune and synovial cells.
- In addition to harming the cells, mitochondrial dysfunction also produces chemicals called DAMPs (damage-associated molecular patterns), which amplify the immunological response.
As a result, joint injury is sustained through a cycle of persistent inflammation. Moreover, the condition is made worse by the overabundance of fibroblast-like synoviocytes (FLS), which penetrate bone and cartilage due to mitochondria’s failure to control normal cell death (apoptosis).
Mitochondrial Dysfunction in Gout
Mitochondrial dysfunction is a major cause of inflammation in gout. The accumulation of uric acid crystals in the joints, which sets off an immunological reaction, is the hallmark of gout. By producing ROS, which triggers the NLRP3 inflammasome, a vital part of the body’s inflammatory response, mitochondria contribute to this process.
This pathway, which is activated by mitochondria, greatly increases inflammation and results in the excruciating pain and swelling that are characteristic of gout attacks.
Mitochondrial Dysfunction in Osteoarthritis
Similarly, decreased energy generation in osteoarthritis (OA) is caused by mitochondrial dysfunction in chondrocytes, the cells responsible for maintaining cartilage, even though OA is not predominantly an autoimmune disease
This contributes to joint deterioration by interfering with cartilage healing processes and quickening the degeneration of cartilage. In addition to causing low-grade inflammation, damaged mitochondria’s abundant ROS exacerbate joint deterioration.
As a result, mitochondria are important in all types of arthritis. Now having gained an understanding of how mitochondrial malfunction contributes to inflammation and joint degradation, researchers and physicians are investigating novel treatment approaches targeted at enhancing mitochondrial health in order to more effectively treat these crippling illnesses.
Triggers of Inflammation in Arthritis
As we’ve looked at the fundamental roles that mitochondrial dysfunction plays in different forms of arthritis, knowing what triggers inflammation and flare-ups is another essential part of controlling these conditions.
Finding the triggers that cause these inflammatory reactions is essential to properly managing both osteoarthritis (OA) and rheumatoid arthritis (RA), conditions where inflammation plays a major role in worsening of symptoms.
Flares in the chronic autoimmune disease RA can happen at any time and have a significant influence on day-to-day living. Symptoms including stiffness, edema, and joint discomfort might intensify during these flare-ups, frequently without prior notice.
Factors such as infections, poor sleep, stress, and overexertion can cause predicted flare-ups of RA. For example, an excessive amount of physical activity or a cold can trigger a flare. Unpredictable flares, on the other hand, are more difficult to manage since they could necessitate modifying medication or therapy regimens and happen for no apparent reason. Inflammation can be made worse by environmental variables like cold weather, variations in barometric pressure, and air pollution. Lifestyle factors like smoking can also make symptoms worse and lead to drug resistance.
However, inflammation can also be a factor in osteoarthritis (OA), which is generally thought to be a degenerative disorder, particularly during flare-ups. Joint traumas, overuse, or repetitive motions can cause flare-ups in osteoarthritis (OA), which can cause pain and stiffness. Changes in the weather and physical and emotional stress can exacerbate symptoms of osteoarthritis
Patients with RA and OA can better control their diseases if they are aware of the specific factors that lead to inflammation. Reducing stress, getting regular exercise, eating anti-inflammatory foods, and maintaining regular contact with healthcare professionals are all lifestyle changes that help lessen the intensity and frequency of flare-ups. Patients are able to manage the ups and downs of arthritis while maintaining a higher quality of life thanks to this proactive strategy.
How to Improve Mitochondrial Health to Reduce Inflammation
After reading this article, you might start to understand the important role that mitochondrial dysfunction plays in arthritis. Because of this it is of great importance to focus on improving mitochondrial health as a strategy to reduce inflammation and manage the symptoms associated with it. Including lifestyle changes such as exercising regularly, eating healthy and balanced as well as managing stress and decreasing cortisol are the best ways to boost your mitochondria´s health.
The importance of eating a balanced diet that is high in antioxidants such as greens, berries, and fatty fish is important because they do a fantastic job fighting oxidative stress. Exercise also is important as it stimulates mitochondrial biogenesis, which is the process by which new mitochondria are formed. Additionally, getting enough sleep and avoiding smoking can support overall cellular health and reduce inflammation.
Read Next
References
- Tiwari V, Bergman MJ. Viral Arthritis. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Viral Arthritis
- Reactive Arthritis. May 2024. National Institute of Arthritis and Musculoskeletal and Skin Diseases. May 2024. Reactive Arthritis.Reactive Arthritis.
- Yap HY, Tee SZ, Wong MM, Chow SK, Peh SC, Teow SY. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells. 2018 Oct 9;7(10):161. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development
- Ames, Hana. 2023, June 28). What’s the difference between inflammatory arthritis and rheumatoid arthritis? MedicalNewsToday. What’s the difference between inflammatory arthritis and rheumatoid arthritis?
- Ma C, Wang J, Hong F, Yang S. Mitochondrial Dysfunction in Rheumatoid Arthritis. Biomolecules. 2022 Sep 1;12(9):1216. Mitochondrial Dysfunction in Rheumatoid Arthritis
- Lakra Promila, Anubha Joshi, Shazia Khan, Amita Aggarwal, Amit Lahiri. Role of mitochondrial dysfunction in the pathogenesis of rheumatoid arthritis: Looking closely at fibroblast- like synoviocytes, Mitochondrion, Volume 73, 2023, Pages 62-71. Role of mitochondrial dysfunction in the pathogenesis of rheumatoid arthritis: Looking closely at fibroblast- like synoviocytes
- Yang SK, Zhang HR, Shi SP, Zhu YQ, Song N, Dai Q, Zhang W, Gui M, Zhang H. The Role of Mitochondria in Systemic Lupus Erythematosus: A Glimpse of Various Pathogenetic Mechanisms. Curr Med Chem. 2020;27(20):3346-3361. The Role of Mitochondria in Systemic Lupus Erythematosus: A Glimpse of Various Pathogenetic Mechanisms
- Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011 Mar;7(3):161-9. doi: 10.1038/nrrheum.2010.213. Epub 2011 Jan 4. The role of mitochondria in osteoarthritis
- Wang Q, Qiu H. Deubiquitinase USP16 induces gouty arthritis via Drp1-dependent mitochondrial fission and NLRP3 inflammasome activation. Arthritis Res Ther. 2023 Jul 24;25(1):126. Deubiquitinase USP16 induces gouty arthritis via Drp1-dependent mitochondrial fission and NLRP3 inflammasome activation